Sustained Killing by Cytotoxic T cells: Mitochondrial Role
News Release, World Mitochondria Society, Berlin - Germany – March 18, 2022
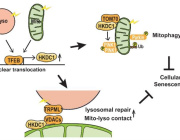
Cytotoxic T lymphocytes (CTLs) can terminate both virally infected cells and cancer cells by secreting cytolytic proteins such as perforin and granzyme B. Lisci et al. have identified mitochondria as important regulators of CTL killing; mice lacking the deubiquitinase USP30 have CTLs acutely depleted of mitochondria, and these cells have reduced killing ability but normal motility, signaling, and secretion.
Although mitochondrial mass has been correlated with CTL antitumor activity, CTLs show an increased reliance on glycolysis, suggesting a decreased dependence on mitochondrial respiration. Whether, how, or why mitochondria contribute as CTLs seek, recognize, and kill their targets is not well understood.
In this research, Lisci et al., generated CTLs from USP30-deficient mice to study the nature of this defect and to understand how it affects killing.
They reported the following:
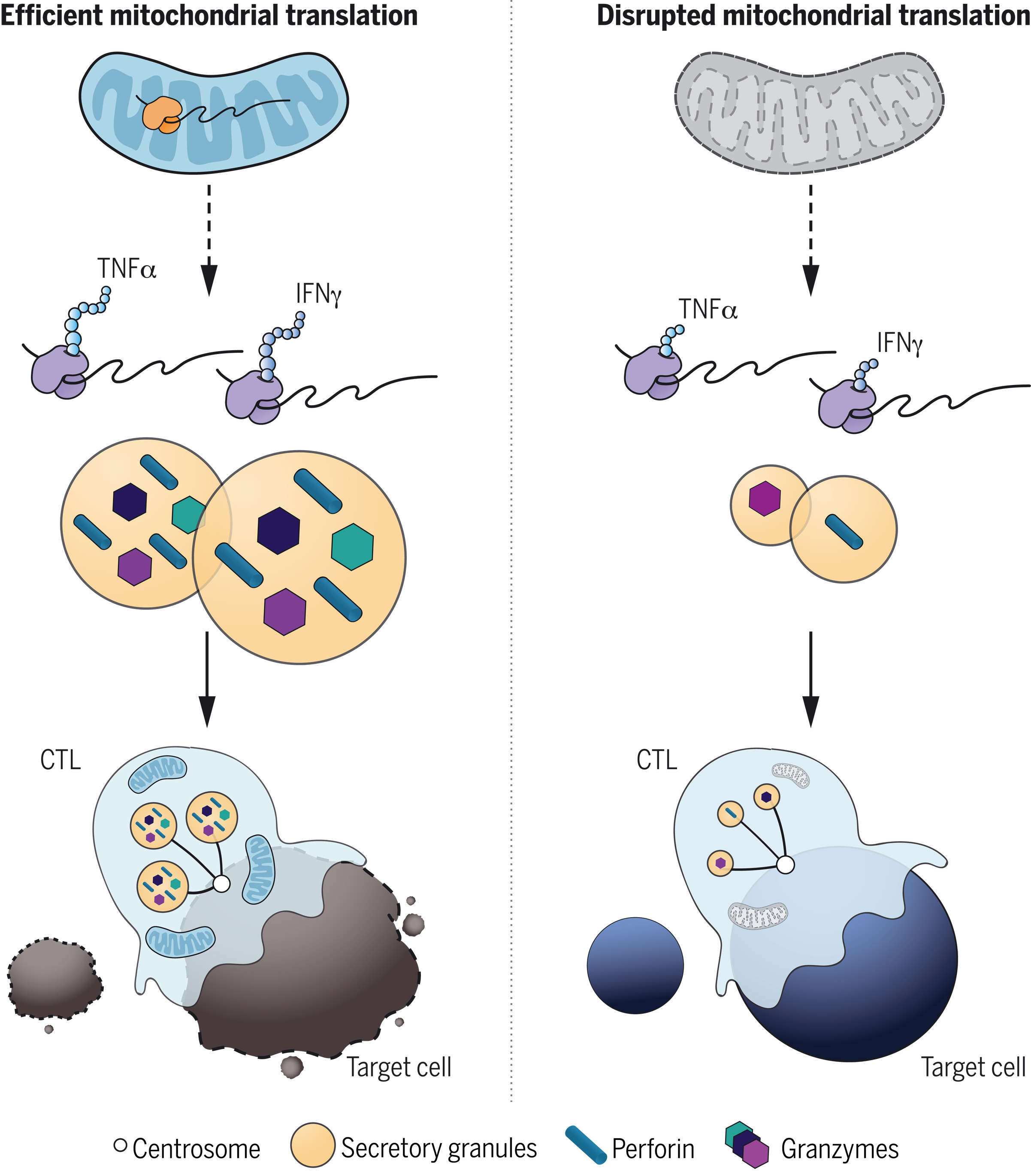
T cell development was unaffected in USP30-deficient mice. However, upon activation, CD8+ T cells generated CTLs with an acute loss of mitochondria and impaired killing.
The cytotoxicity of USP30-deficient CTLs diminished with time, indicating a defect in sustained killing.
Despite the loss of mitochondria and decreased oxidative phosphorylation in Usp30−/− CTLs, motility, signaling, and secretion, which are required for killing, were all intact. However, the secretory granule size was reduced in Usp30−/− CTLs, with a reduction in newly synthesized intermediates of key cytolytic proteins, perforin and granzyme B. This suggested an underlying defect in the de novo protein synthesis, which is required for sustained killing.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com