Could fixing mitochondria restore insulin-producing cells?
Mitochondria - the tiny power plants inside our cells may be a master switch in diabetes, controlling not just energy production but the very identity of key metabolic cells.
Core finding:
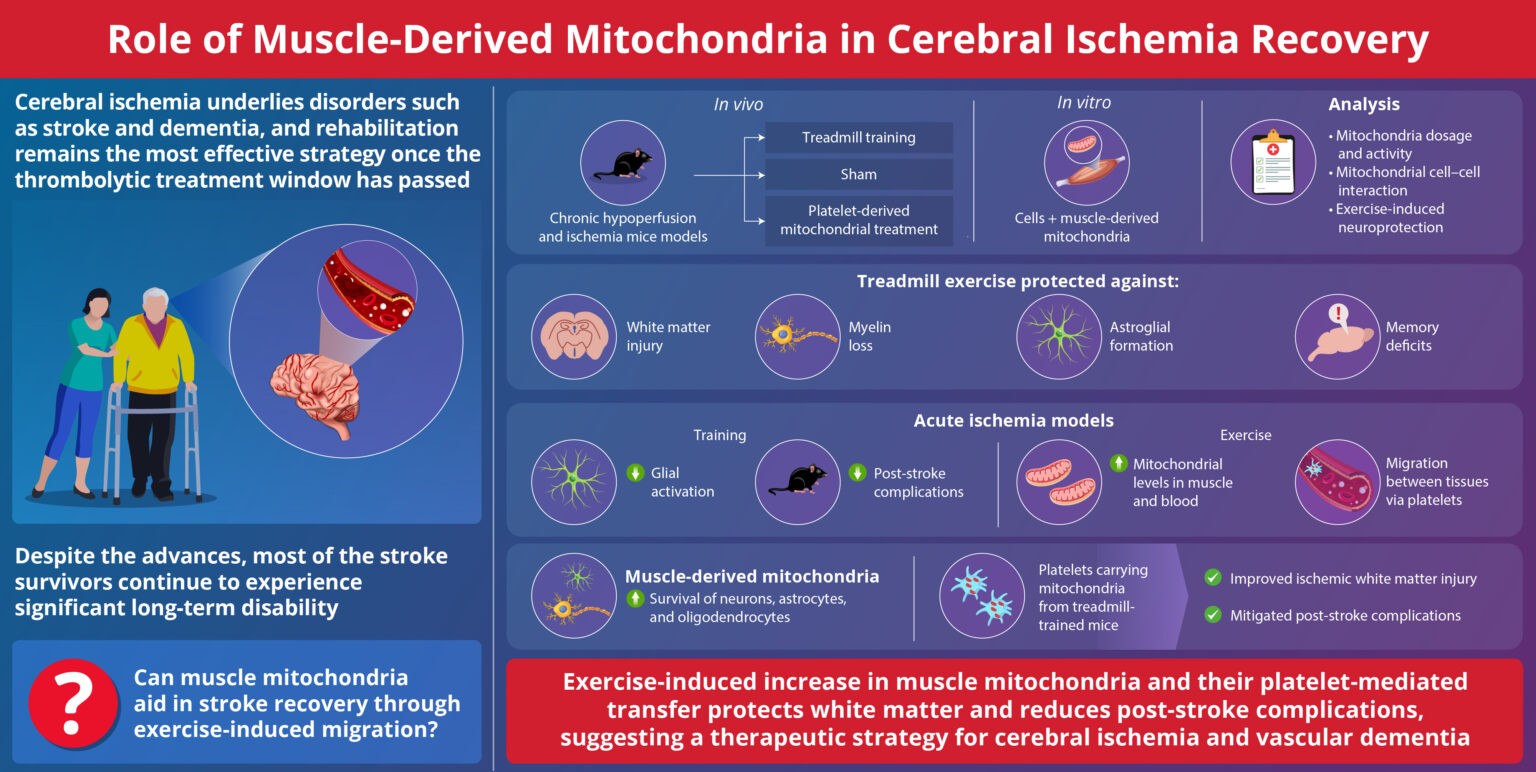
University of Michigan researchers lead by EMILY M. WALKER and team show that when mitochondria are damaged in insulin-producing pancreatic β-cells, a specific cellular stress response switches on, causing these cells to become “immature,” produce too little insulin, and essentially stop functioning as true β-cells. Crucially, the team finds that this same mitochondrial stress program can also be triggered in liver and fat cells, hinting at a unifying mechanism behind the multi-organ breakdown seen in diabetes.
How they tested it::
Using mice, the scientists disrupted three separate pillars of mitochondrial health: mitochondrial DNA, the pathway that clears damaged mitochondria, and the system that keeps a healthy pool of mitochondria in the cell. Despite these different hits, each manipulation triggered the exact same stress response and led β-cells to lose maturity and insulin output, revealing a common “danger signal” from mitochondria to the cell nucleus.
Beyond the pancreas:
Because diabetes affects far more than the pancreas, the team repeated the experiments in liver cells and fat-storing cells, again seeing the same stress response and impaired cell maturation and function. Senior author Scott Soleimanpour notes that losing β-cells is the most direct route to type 2 diabetes, but these results suggest a shared mitochondrial glitch across tissues could be the deeper root cause.
A possible way to reverse it:
In a hopeful twist, mitochondrial damage did not kill the cells, raising the possibility that their function could be restored. When the researchers treated mice with ISRIB, a drug that blocks the stress response pathway, β-cells regained their ability to control blood sugar within four weeks, effectively reversing the mitochondrial-driven dysfunction in this model.
What’s next:
The team confirmed key findings in human pancreatic islet cells and is now dissecting the disrupted pathways in more detail, aiming to repeat the rescue in cells from people with diabetes. If successful, targeting this mitochondrial stress signaling could shift diabetes treatment from managing blood sugar to correcting a fundamental cellular error – turning the cell’s “powerhouse” into a therapeutic entry point.
Click here for more information.
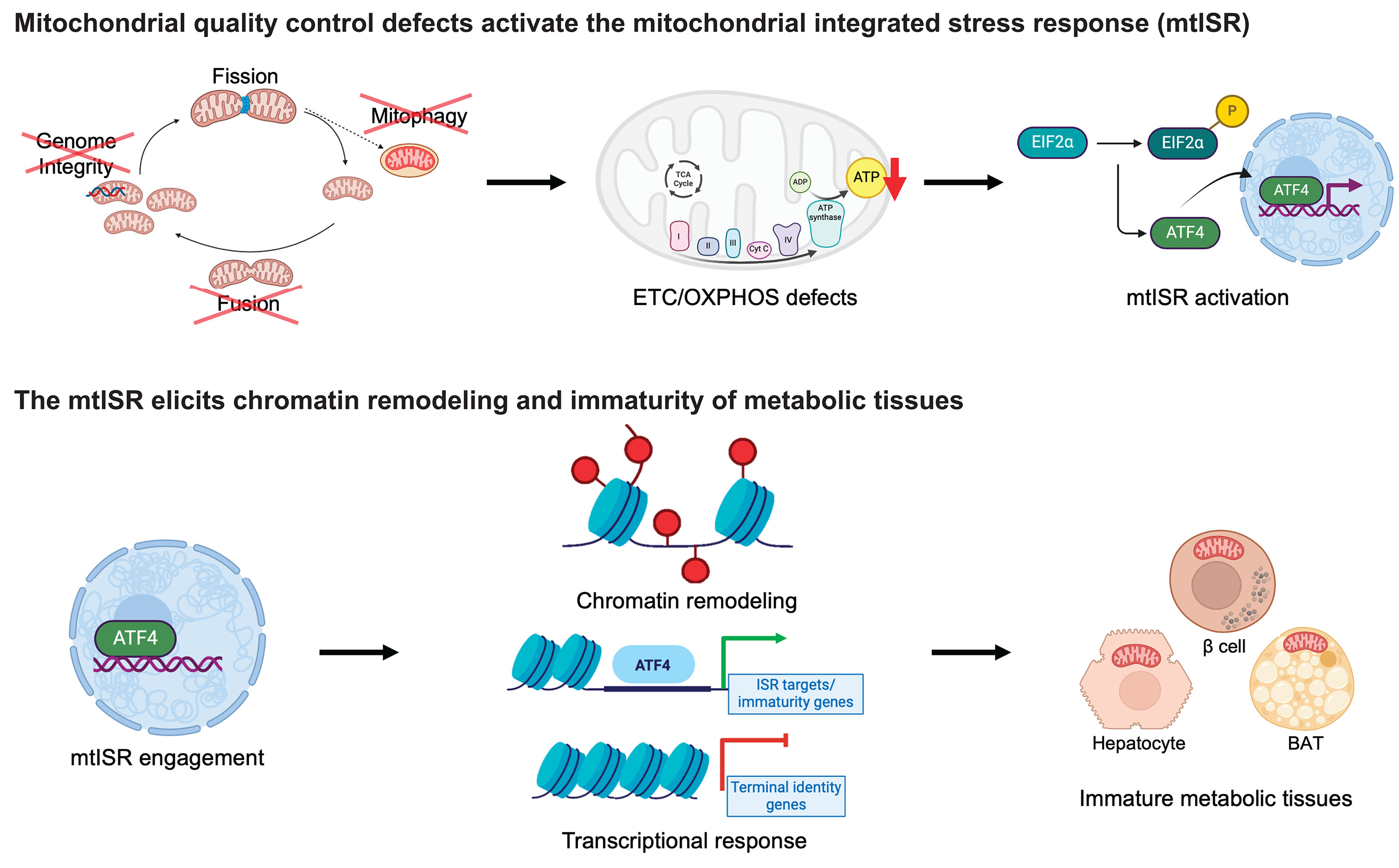
About Image
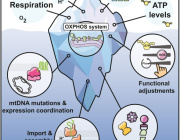
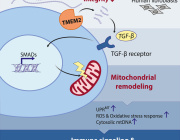
A retrograde mitochondrial signaling cascade induces the loss of identity and maturity in metabolic tissues.
Numerous defects in the mitochondrial quality control machinery are capable of eliciting defects in the ETC-OXPHOS system, which acts as a retrograde signal to trigger the mtISR. Engagement of the mtISR induces chromatin remodeling and transcriptional and functional immaturity in metabolic tissues. TCA, tricarboxylic acid; ADP, adenosine 5′-diphosphate; ATP, adenosine 5′-triphosphate; BAT, brown adipose tissue. [Figure created with BioRender.com]
Referance
DOI: 10.1126/science.adf203
We are pleased to announce that the 17th Conference Targeting Mitochondria 2026 will be held in Berlin, Germany, from October 21-23. We look forward to welcoming you.