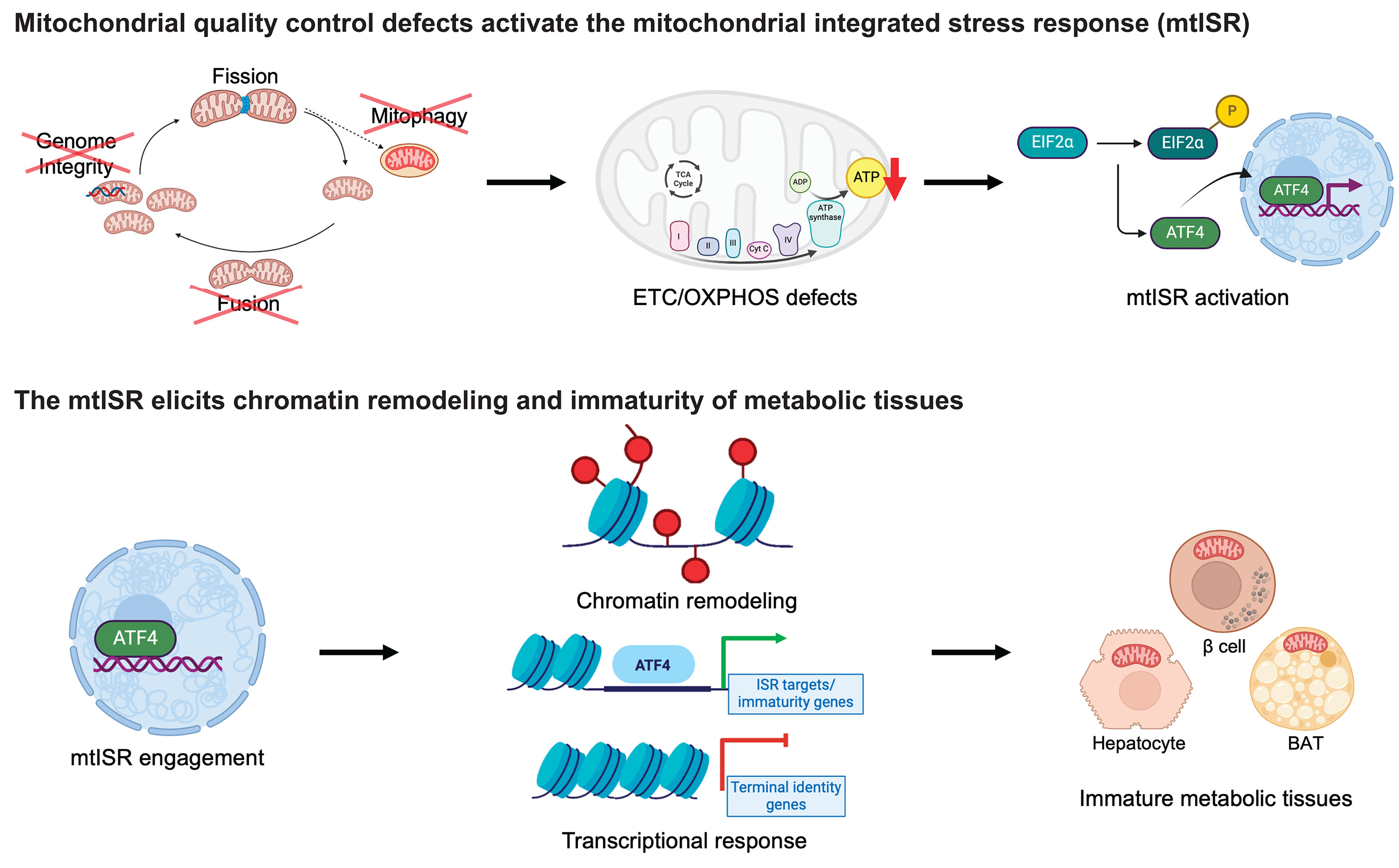
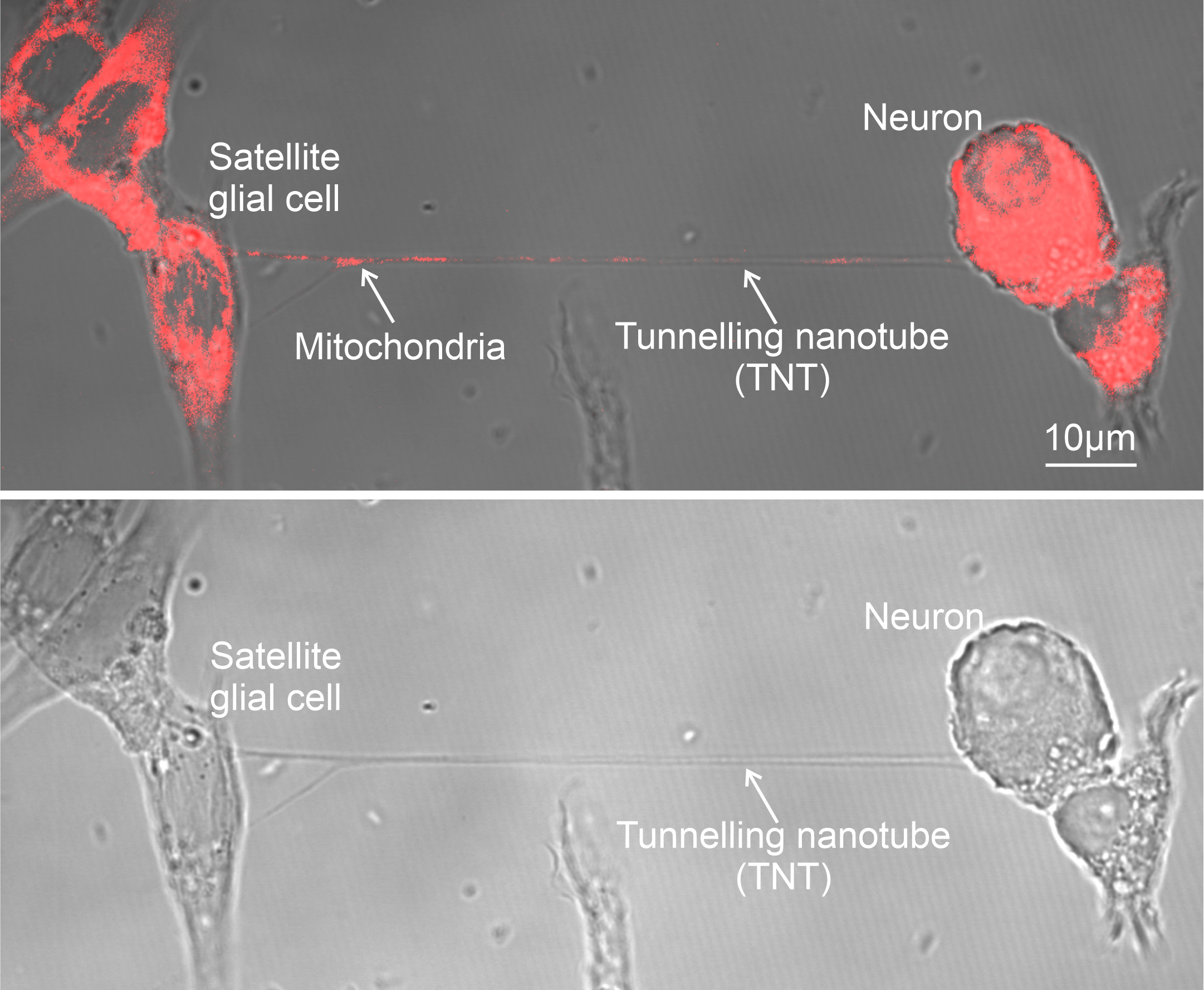
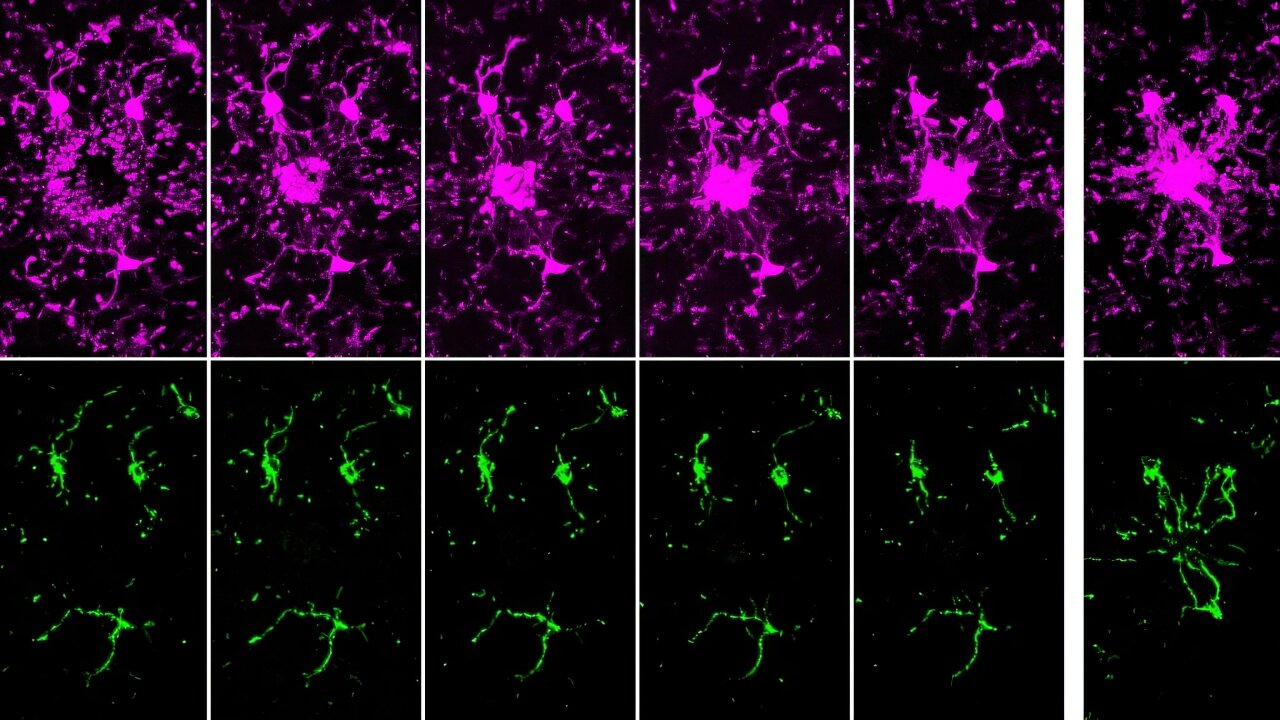
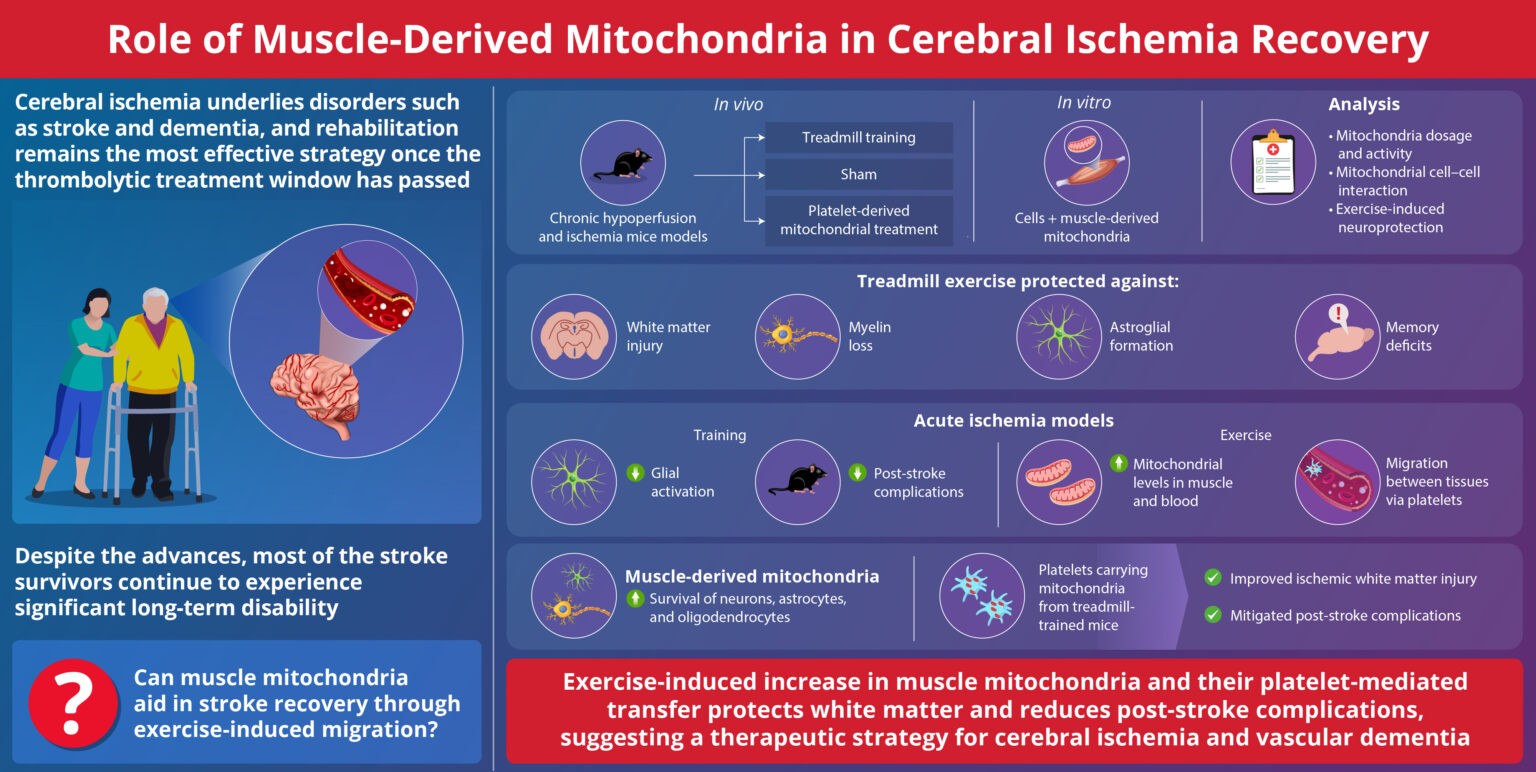
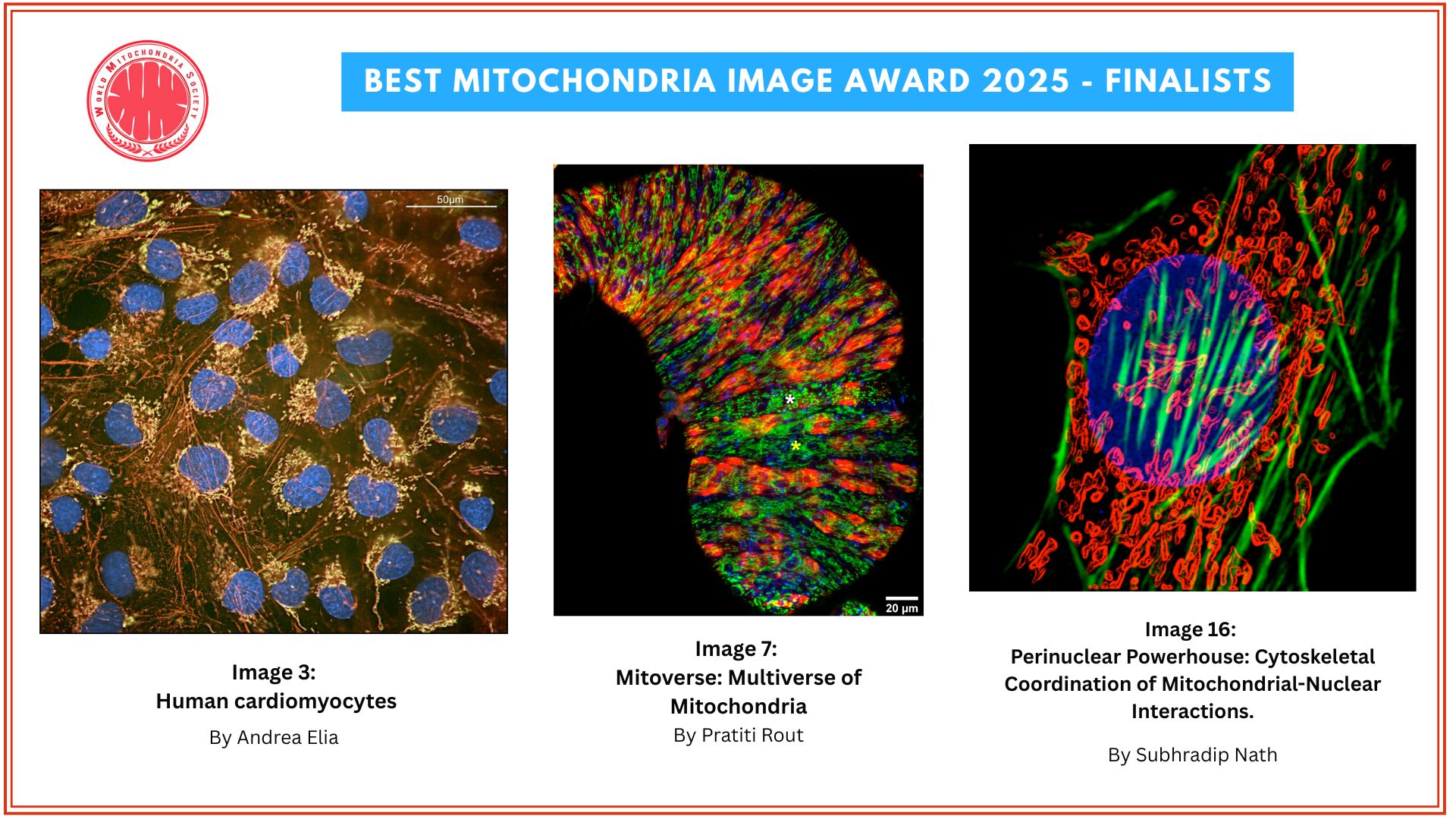
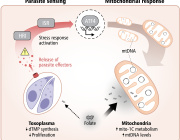
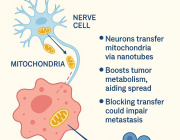
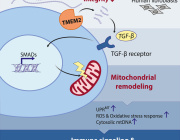
Mitochondria Organelle Transplantation for Neurological Diseases & Aging
 Dr. Mark S. Kindy from the University of South Florida, USA, will join the Targeting Mitochondria 2023 Congress and give a presentation entitled "Mitochondria Organelle Transplantation for Neurological Diseases and Aging".
Dr. Mark S. Kindy from the University of South Florida, USA, will join the Targeting Mitochondria 2023 Congress and give a presentation entitled "Mitochondria Organelle Transplantation for Neurological Diseases and Aging".
Join Targeting Mitochondria 2023 to learn more about Dr. Kindy's exciting talk.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
What spaceflight and bed rest have in common: A proteomic point of view
 Dr. Marta Murgia from the University of Padova, Italy will join the Targeting Mitochondria 2023 Congress and give a presentation entitled "What spaceflight and bed rest have in common: A proteomic point of view".
Dr. Marta Murgia from the University of Padova, Italy will join the Targeting Mitochondria 2023 Congress and give a presentation entitled "What spaceflight and bed rest have in common: A proteomic point of view".
In the absence of mechanical loading, skeletal muscle undergoes atrophy with loss of strength and detrimental metabolic effects. Dr. Murgia and her team used highly sensitive mass spectrometry (MS)-based proteomics to detail single fiber type-specific molecular remodeling caused by muscle unloading, using bed rest as a model.
In parallel, they measured the muscle proteome of two astronauts, from biopsies taken before and after a six months mission on the International Space Station (ISS). In their muscle lysates, they measured a sharp decrease in the expression of the whole mitochondrial proteome.
Dr. Murgia's talk will give an overview of the main proteomic changes that we measured in these two different models of muscle unloading and disuse. She will highlight striking similarities as well as profound differences. She will discuss the protective role of exercise on skeletal muscle mass in space, with a focus on the mitochondrial proteome.
Join Targeting Mitochondria 2023 to learn more about Dr. Murgia's exciting talk. You can submit a related abstract here.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Advances in the Development of Mitochondria-Targeted Pharmaceuticals
 Dr. Maxim Skulachev, CSO at Mitotech (Israel) , who manages Mitotech's core scientific research, will share the latest advances in developing mitochondria targeting drugs during Targeting Mitochondria 2023 this October.
Dr. Maxim Skulachev, CSO at Mitotech (Israel) , who manages Mitotech's core scientific research, will share the latest advances in developing mitochondria targeting drugs during Targeting Mitochondria 2023 this October.
In the last 15 years of his life and scientific career, Vladimir Skulachev dedicated himself to his project on practical application of penetrating ions. This ambitious endeavor focuses on developing new pharmaceuticals based on mitochondria-targeted antioxidants of the SkQ class.
Mitotech's leading compound, SkQ1, is currently undergoing extensive development for different indications and in various pharmaceutical forms, including eye drop formulations (which have reached the third stage of clinical trials in the US), as well as oral and injectable "systemic" formulations.
In this presentation, Dr. Skulachev would like to share their recent findings from preclinical studies they completed using the latter formulations, Mitotech team successfully harnessed both the antioxidant and mild uncoupling properties of SkQ1 molecule.
Dr. Skulachev's short talk will be titled: "Advances in the Development of Mitochondria-Targeted Pharmaceuticals".
Read more about Professor Vladimir Skulachev's impact.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Vladimir Skulachev's Strategic Impact on Mitochondrial Medicine: A Tribute to his Vision, Discoveries, and Legacy
 Prof. Vladimir Gogvadze, Karolinska Institutet, Sweden, and active member of the scientific committe will join Targeting Mitochondria 2023 this October and introduce the congress.
Prof. Vladimir Gogvadze, Karolinska Institutet, Sweden, and active member of the scientific committe will join Targeting Mitochondria 2023 this October and introduce the congress.
Prof. Gogvadze will dedicate his talk to Prof. Vladimir Skulachev, former member of the scientific committee of WMS, to recognize his impact in the Mitochondria World: "Vladimir Skulachev's Strategic Impact on Mitochondrial Medicine: A Tribute to his Vision, Discoveries, and Legacy".
Read more about Professor Skulachev's impact.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Metabolic effects of Cimicfuga racemosa extract on mitochondria and implications for the resistance against oxidative cell death and longevity
 Prof. Carsten Culmsee, active member of the scientific committe and from the University of Marburg, Germany, will join Targeting Mitochondria 2023 this October. He will have a talk entitled "Metabolic effects of Cimicfuga racemosa extract on mitochondria and implications for the resistance against oxidative cell death and longevity".
Prof. Carsten Culmsee, active member of the scientific committe and from the University of Marburg, Germany, will join Targeting Mitochondria 2023 this October. He will have a talk entitled "Metabolic effects of Cimicfuga racemosa extract on mitochondria and implications for the resistance against oxidative cell death and longevity".
Cimicifuga racemosa extract (CRE) is a well-established herbal medication to treat menopausal symptoms such as hot flashes and weight gain. In contrast to estrogen replacement therapy or phytoestrogens, however, CRE does not act through stimulation of estrogen receptors but through metabolic mechanisms. Prof. Culmsee and his team's findings suggest that CRE Ze 450 rather exerts direct effects on mitochondrial energy turnover through interference with components of the mitochondrial electron transport chain (ETC).
Prof. Culmsee will provide a comprehensive insight into the signalling effects of the extract on the mitochondrial proteome and metabolome, highlighting the role of CREe for the resilience against age-related processes engaging impaired mitochondria and loss of antioxidative capacities.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
 Dr. Paul Hwang, Senior Investigator at the National Heart, Lung and Blood Institute, USA will present his latest finding targeting mitochondria in chronic fatigue symptoms during Targeting Mitochondria 2023 this October.
Dr. Paul Hwang, Senior Investigator at the National Heart, Lung and Blood Institute, USA will present his latest finding targeting mitochondria in chronic fatigue symptoms during Targeting Mitochondria 2023 this October.
Dr. Hwang's talk will be titled: "WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in ME/CFS". He will share his latest findings reported in the Proceedings of the National Academy of Sciences. Read more about this outstanding publication.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disorder characterized by various disabling symptoms including exercise intolerance. Dr. Hwang and his team report that overexpression of Wiskott-Aldrich Syndrome Protein Family Member 3 (WASF3), here identified in a 38-y-old woman suffering from long-standing fatigue and exercise intolerance, can disrupt mitochondrial respiratory supercomplex formation.
Increased expression of WASF3 in transgenic mice decreased their treadmill running capacity and specific respiratory complexes. Expanding on our findings in a single patient, skeletal muscle biopsy samples obtained from a cohort of patients with ME/CFS showed increased WASF3 protein levels associated with aberrant ER stress activation. Pharmacologic inhibition of ER stress decreased WASF3 and improved mitochondrial function in the cells of the patient with chronic fatigue, suggesting a therapeutic strategy for ME/CFS treatment.
About Dr. Hwang
Dr. Paul Hwang earned B.A. degrees in biochemistry and chemistry from the University of Kansas in 1985, after which he spent a year at the Swiss Federal Institute of Technology and University of Zurich as a Fulbright Scholar. He graduated from the Johns Hopkins University School of Medicine with an M.D. and Ph.D in 1993. He did his internship and residency in internal medicine at the UCSF School of Medicine in San Francisco, followed by a clinical fellowship in cardiology and postdoctoral research in molecular oncology at the Johns Hopkins University School of Medicine. Upon completion of his training in 2001, Dr. Hwang joined the NHLBI-NIH as an investigator and was tenured in 2011. He has been elected as member of the American Society for Clinical Investigation and fellow of the American College of Cardiology.
Join Targeting Mitochondria 2023 to learn more about Dr. Hwang's research.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Mitochondrial Monitoring in Perioperative and Critical Care: Recent Advances & Perspectives
 Dr. Egbert Mik, Erasmus MC, The Netherlands, and ative member or WMS scientific committee, will be joining Targeting Mitochondria 2023. Dr. Mik will give an update on "Mitochondrial Monitoring in Perioperative and Critical Care"
Dr. Egbert Mik, Erasmus MC, The Netherlands, and ative member or WMS scientific committee, will be joining Targeting Mitochondria 2023. Dr. Mik will give an update on "Mitochondrial Monitoring in Perioperative and Critical Care"
In order not to miss this outstanding talk, so join Targeting Mitochondria 2023 to learn more about Dr. Mik's interesting findings.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Brain Organoids to Model Mitochondrial Neurological Diseases
 Prof. Alessandro Prigione, ative member or WMS scientific committee, Heinrich Heine University (HHU) Düsseldorf, Germany, will join Targeting Mitochondria 2023 to present a talk entitled: "Brain Organoids to Model Mitochondrial Neurological Diseases".
Prof. Alessandro Prigione, ative member or WMS scientific committee, Heinrich Heine University (HHU) Düsseldorf, Germany, will join Targeting Mitochondria 2023 to present a talk entitled: "Brain Organoids to Model Mitochondrial Neurological Diseases".
Join Targeting Mitochondria 2023 to learn more about Prof. Prigione's interesting findings. You can submit a related abstract here.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Cholesterol: Why Have Mitochondrial Biologists Ignored this Critical Mitochondrial Component for Over a Century?
 Prof. Ian Holt, active member of the scientific committee, Instituto de Investigación Sanitaria Biodonostia, Spain, will join Targeting Mitochondria 2023 to present a talk titled: "Cholesterol: Why Have Mitochondrial Biologists Ignored this Critical Mitochondrial Component for Over a Century?".
Prof. Ian Holt, active member of the scientific committee, Instituto de Investigación Sanitaria Biodonostia, Spain, will join Targeting Mitochondria 2023 to present a talk titled: "Cholesterol: Why Have Mitochondrial Biologists Ignored this Critical Mitochondrial Component for Over a Century?".
As Michael Brown noted in his 1985 Nobel Lecture: Cholesterol is the most highly decorated small molecule in biology. However, cholesterol’s contribution to mitochondrial membranes has attracted little interest, as they are ‘cholesterol-poor organelles’ with 0.5-3% of the content found in the plasma membrane. And although high cholesterol has been linked to mitochondrial dysfunction, this merely implied that mitochondria have an aversion to cholesterol.
The first forays into this field came with the unexpected discovery that pathological mutant forms of the trans-mitochondrial membrane protein, ATAD3, completely reconfigure cellular cholesterol metabolism.
During Targeting Mitochondria 2023, Prof. Holt will report the central role of cholesterol in the ATAD3 disease cascade, and crucially show that the molecular phenotypes stem from the mitochondrion’s absolute requirement for cholesterol.
Join Targeting Mitochondria 2023 to learn more about Prof. Holt's interesting findings.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Mitochondrial Presequence Protein Translocation
 Prof. Nils Wiedemann, Universität Freiburg, Germany, will join Targeting Mitochondria 2023 to present a talk entitled: "Mitochondrial Presequence Protein Translocation".
Prof. Nils Wiedemann, Universität Freiburg, Germany, will join Targeting Mitochondria 2023 to present a talk entitled: "Mitochondrial Presequence Protein Translocation".
Virtually all of the ~1,000 different mitochondrial proteins are synthesised in the cytosol and must be imported into the organelle. Most of these mitochondrial precursor proteins contain an amino-terminal presequence, which forms a positively charged amphiphilic alpha-helix. The TIM23 translocase sorts these presequence proteins into the inner membrane or matrix. Prof. Wiedemann and colleagues mapped the interaction of the essential subunit Tim17 with presequence containing precursor proteins.Tim17 contains conserved negative charged residues close to the intermembrane space side of the inner membrane, which are essential for presequence protein translocation along a distinct transmembrane cavity of the Tim17-bilayer interface.
About Prof. Wiedemann,
Professor (apl.) of Biochemistry and Molecular Biology, University of Freiburg, Germany
Prof. Wiedmann won the Young Investigator Award, German Society for Biochemistry and Molecular Biology (GBM) Frankfurt in the year 2007.
Join Targeting Mitochondria 2023 to learn more about Prof. Wiedemann's interesting findings. Read more about Prof. Wiedmann's latest research.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Dr. Naig Gueguen announces the Agenda of the Upcoming Workshop on Mitochondrial Function and Dysfunction Evaluation
In association with the World Mitochondria Society (WMS), we are thrilled to announce the hosting of a specialized workshop focused on the Evaluation of Mitochondria Function, Dysfunction, and Activities. The workshop will take place on October 11, 2023, one day prior to the start of the Targeting Mitochondria 2023 Congress.
Purpose of the Workshop
This workshop will delve into the significant role mitochondria play in various medical fields, including cardiology, neurology, nephrology, and infectious diseases, among others. Given the centrality of mitochondria to human metabolism, their assessment is vital for the discovery of treatments for diseases associated with mitochondrial dysfunction.
Workshop Key Objectives
- To introduce and review technological tools used for mitochondrial study and analysis.
- To provide an understanding of mitochondrial markers evaluated in the context of medical research.
- To present various methodologies for analyzing mitochondria and their functions, including bioenergetics, biogenesis, dynamics, and mitophagy.
- To discuss biochemical diagnostic strategies for mitochondrial diseases.
- To discuss practical cases to solidify understanding.
Who Can Attend?
The workshop welcomes everyone looking to enhance their understanding and proficiency in the field of mitochondria, metabolism, and bioenergetics, their vital roles within the human body, as well as the latest innovations in therapeutic tools and analyses.
About Dr. Naig Gueguen:
 Dr. Gueguen is an expert from the Mitovasc Institute, Mitolab team, and member of INSERM 1083, CNRS 6215, Centre Hospitalier Universitaire d’Angers. She will eagerly anticipate this enriching workshop. The Mitovasc Institute's Mitolab Team is renowned worldwide for its work in mitochondrial research. The team focuses on studying mitochondrial function and dysfunction, with the aim of developing effective treatments for various diseases.
Dr. Gueguen is an expert from the Mitovasc Institute, Mitolab team, and member of INSERM 1083, CNRS 6215, Centre Hospitalier Universitaire d’Angers. She will eagerly anticipate this enriching workshop. The Mitovasc Institute's Mitolab Team is renowned worldwide for its work in mitochondrial research. The team focuses on studying mitochondrial function and dysfunction, with the aim of developing effective treatments for various diseases.
Contact WMS: This email address is being protected from spambots. You need JavaScript enabled to view it.
Targeting Mitochondrial Activity in Liver in Disease: Barriers and Perspectives
 Prof. María Luz Martínez-Chantar, CIC bioGUNE, Spain, will join Targeting Mitochondria 2023 to present a talk entitled: "Targeting Mitochondrial Activity in Liver in Disease: Barriers and Perspectives".
Prof. María Luz Martínez-Chantar, CIC bioGUNE, Spain, will join Targeting Mitochondria 2023 to present a talk entitled: "Targeting Mitochondrial Activity in Liver in Disease: Barriers and Perspectives".
Mitochondrial dysfunction is a critical factor contributing to the pathogenesis and progression of chronic liver diseases. This study conducted by Prof. Martínez-Chantar aims to elucidate key players, causative factors, and consequences of mitochondrial dysfunction in the context of liver health.
Specifically, she and her team explore the potential therapeutic benefits of enhancing mitochondrial activity by modulating genes related to the electron transport chain, mitochondrial metabolism and cation modulators in the liver.
The findings provide compelling evidence supporting different genes as a promising therapeutic approach that not only ameliorates liver injury but also fosters liver regeneration.
Join Targeting Mitochondria 2023 to learn more about Prof. Martínez-Chantar's interesting findings.
About Prof. Martinez-Chantar
Professor Martinez-Chantar has an extensive experience in the study of liver biology and disease with a high-level track of productivity in the 1st decile journals like Nature Communications, Cell Metabolism, Hepatology, Journal of Hepatology and Gastroenterology. She has been continuously supported by competitive public and private funding, both national and international, including NIH. She coordinates the Translational Area of the National Institute for the study of Liver & Gastrointestinal Diseases and is in the SAB of the Molecular Medicine Center Nice, IDIVAL and IDIBAPS. She shows extensive participation in different networks (CibereHD, Women in Hepatology: International Consortium, Hepamet Registry, MetaboCancer Excellence Network and diverse EU COST actions). Her contracts with pharmas, as AGIOS, Mitotherapeutix, Takeda or Silence Therapeutics, led to 5 patent applications and 4 licensed products. Her collaboration with OWL Metabolomics led to the development of OWLiver® Care and OWLiver®, non-invasive assays for NASH diagnosis.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Targeting Mitochondria Based on Mitochondrial Drug Delivery Systems (DDS)

Under its guiding slogan “The future of medicine will come through mitochondria”, the World Mitochondria Society proudly presents Targeting Mitochondria 2023 speakers. This conference gathers the brightest minds, leaders and actors in mitochondrial research.
At the forefront of this gathering is Prof. Yuma Yamada of the Faculty of Pharmaceutical Sciences, Hokkaido University, who will give a standout presentation on “Targeting Mitochondria Based on Mitochondrial Drug Delivery Systems (DDS).” Prof. Yamada’s recent recognition as the recipient of the American Pharmacists Association’s 2022 Ebert Prize—the oldest and one of the most prestigious pharmacy awards in the US—further cements his standing in the field.
A Significant Advancement in Mitochondrial Research: The MITO-Porter
Addressing challenges that have spanned a decade in mitochondrial drug delivery, Prof. Yamada’s development of the MITO-Porter is especially noteworthy. This cutting-edge nano DDS is meticulously engineered to transport macromolecular cargos directly into mitochondria via membrane fusion. The MITO-Porter represents not just a technological marvel but a beacon for the future of mitochondrial medicine.
Although various mitochondrial drug delivery systems have emerged over the years, many have encountered hurdles. The MITO-Porter, with its groundbreaking approach, navigates these challenges, heralding a new era of therapeutic potential.
In his presentation, Prof. Yamada will provide an in-depth overview of the MITO-Porter, elucidating its design, capabilities, and forward-looking applications. Attendees will be enriched with insights into the contemporary landscape of mitochondrial DDS and will experience the profound impact of the MITO-Porter in the domain of mitochondrial therapy.
Join Targeting Mitochondria 2023 to learn more about Prof. Yamada's interesting talk.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Mitochondrial Transplantation: What's Next?
 Dr. James D. McCully, from The Harvard Medical School Department of Cardiac Surgery Boston Children’s Hospital, USA, will join us this year to present his most recent findings: "Mitochondrial Transplantation What's Next?".
Dr. James D. McCully, from The Harvard Medical School Department of Cardiac Surgery Boston Children’s Hospital, USA, will join us this year to present his most recent findings: "Mitochondrial Transplantation What's Next?".
Join Targeting Mitochondria 2023 to learn more about Dr. McCully's interesting talk.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
Why a WMS Task Force?
 Prof. Marvin Edeas, Institut Cochin, Université de Paris, France, will join Targeting Mitochondria 2023 to explain further on : "Why a WMS Task Force?".
Prof. Marvin Edeas, Institut Cochin, Université de Paris, France, will join Targeting Mitochondria 2023 to explain further on : "Why a WMS Task Force?".
Join Targeting Mitochondria 2023 to learn more about Prof. Edeas's interesting talk.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany